Trulance VS Linzess (Plecanatide Vs Linaclotide) are two novel medicines that are being increasingly used to treat patients with chronic idiopathic constipation and Irritable bowel syndrome with predominant symptoms of constipation.
Trulance Vs Linzess (Plecanatide Vs Linaclotide)
What is Trulance?
Trulance is the brand name of Plecanatide. It relieves constipation by acting as a guanylate cyclase agonist. It increases intestinal fluid by causing the secretion of chloride and bicarbonate into the intestinal lumen. It also relieves visceral abdominal pain and increases intestinal transit.
It is indicated for the treatment of chronic constipation without a known cause.
Read: Treatment of Constipation with novel agents
What is Linzess?
Linzess is the brand name of Linaclotide. It has a similar mechanism of action as Trulance (Plecanatide). It is a guanylate cyclase agonist that increases the intracellular and extracellular levels of cyclic guanosine monophosphate (cGMP). The drug increases intestinal transit, relieves abdominal visceral pain, and increases intestinal fluid by activating intraluminal chloride and bicarbonate.
It is indicated for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation.
Read: Treatment of IBS with novel agents
How are Trulance and Linzess Different?
There are minimal differences between the two drugs. The following table is a summary of the comparison of Trulance vs Linzess:
Trulance | Linzess | |
Generic Names | Plecanatide | Linaclotide |
FDA approval | 2017 | 2012 |
Manufactured by | Synergy Pharmaceuticals | Ironwood pharmaceuticals |
Indications | Approved in chronic idiopathic constipation | Approved in chronic idiopathic constipation and irritable bowel syndrome with constipation |
Dosage forms | Available as 3 mg capsules | Available as 72 mcg, 145 mcg, and 290 mcg capsules |
Doses | 3 mg once daily | Chronic idiopathic constipation: 72 mcg to 145 mcg once daily IBS-C: 290 mcg once daily |
Metabolism and excretion | It is metabolized into its active moiety and degraded into peptides in the intestinal lumen before excretion | It is metabolized into its active moiety and degraded into peptides in the intestinal lumen before excretion. |
Efficacy in Clinical trials | Plecanatide was studied in patients with CIC (chronic idiopathic constipation) in two clinical trials. 21% of the patients in the Plecanatide group responded at least 9 out of 12 weeks (and 3 out of the last four weeks) of the study period compared to 10 – 13 % in the placebo groups. | In clinical trials, abdominal pain improved in 34 – 39% of the patients compared to 20 -27% in the placebo groups. CSBM (complete spontaneous bowel movements) occurred in 18 to 20% of the patients in the linaclotide group compared to 5 – 6% in the placebo groups. Combined symptoms (abdominal pain and CSBM) improved in 12 – 13 % of the patients in the linaclotide groups compared to 3 – 5% in the placebo groups. |
Side effects of Trulance Vs Linzess
What are the Possible Side Effects of Trulance?
Trulance (Plecanatide) acts in the intestinal lumen and does not get absorbed. Since it causes the secretion of fluid rich in bicarbonate and chloride, it can cause diarrhea. In fact, diarrhea is the most common side effect of Plecanatide. Patients who may be at risk of developing severe dehydration should not be prescribed this medicine.
It is contraindicated in patients who are six years of age or younger because of the risk of severe dehydration and death.
The Most common Side effect of Trulance is Diarrhea
In clinical trials, diarrhea occurred in 5% of the patients in the Plecanatide group compared to 1% in the placebo group. Severe diarrhea occurred in 0.6% in the Plecanatide group compared to 0.3% in the placebo group.
Other less common side effects that were observed in the clinical trials included:
- Gastrointestinal-related:
- Abdominal pain, flatulence, abdominal distension, and abdominal tenderness,
- Infections:
- sinusitis, pharyngitis, and respiratory tract infections.
- Hepatobiliary-related:
- Hepatotoxicity manifesting as raised liver enzymes were also observed.
What are the Possible Side Effects of Linzess?
Linzess or linaclotide acts inside the intestinal lumen. Since it is primarily used to treat constipation, diarrhea is the most commonly reported adverse reaction of linaclotide.
It is contraindicated in individuals at risk of developing severe dehydration and children who are 6 years of age or younger.
Diarrhea occurred in 2% of the patients who were treated for chronic constipation when the drug was administered in a dose of 145 mcg daily. Less than 1% of the patients developed diarrhea when the drug was administered in a dose of 72 mcg daily.
The most common side effect of Linzess is Diarrhea
In clinical trials, diarrhea resulting in electrolyte imbalance, dehydration, syncope, dizziness, and hypotension were reported that required hospitalization.
Patients in the IBS-C trial, who were treated with 290 mcg once daily Linzess, the following percentage of patients reported adverse drug reactions:
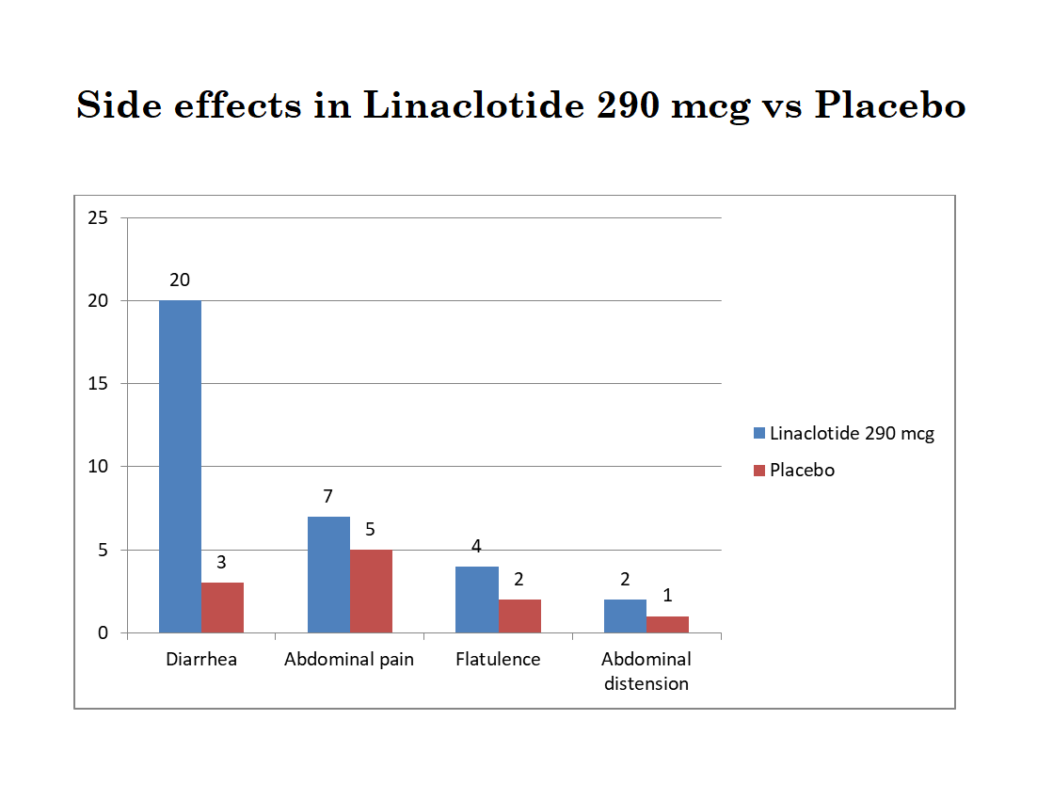
Diarrhea occurred in 20% of the Linaclotide-treated patients compared to 3% of the placebo-treated patients. Out of these patients, severe diarrhea occurred in 2% of the linaclotide-treated patients compared to 1% of the placebo-treated patients.
Less common (<2%) of the patients developed fecal incontinence, fecal urgency, vomiting, and GERD (gastroesophageal reflux disease).

Conclusion:
Linaclotide and Plecanatide are two novel drugs that are used to treat patients with chronic constipation of no known cause. Linaclotide is also used to treat patients with IBS-C (irritable bowel syndrome with constipation). Both drugs act inside the intestinal lumen and may be associated with severe diarrhea especially in children and elderly patients.