Milnacipran is a selective serotonin and nor-epinephrine reuptake inhibitor (SNRI). It is available by the brand names of Ixel, Joncia, and Savella, among others. Like other SNRIs, it can be used to treat depression and fibromyalgia.
Although many clinical trials have confirmed the drug’s efficacy in the treatment of depression, it has not yet been approved by the FDA for the treatment of depression. It has been approved for the treatment of fibromyalgia. Fibromyalgia is a condition manifesting as body pains, fatigue, lethargy, and depression. It is probably a subset of depression where the patients mostly have somatic symptoms rather than psychological symptoms.
Milnacipran (Ixel) has not been approved by the FDA for Depression
Milnacipran (Ixel) has been approved for the treatment of Fibromyalgia
Milnacipran (Ixel) Dose and Administration:
Milnacipran is administered twice daily. The treatment is initiated at a dose of 12.5 mg two times a day and then increased to 25 mg two times a day on the third day. If the patient develops any side effects after the dose has been increased, the low dose can be continued for a week or two and then increased if the symptoms of depression and fibromyalgia has not improved by at least 30 to 50%.
The usual recommended dose is 50 mg twice daily. Patients are titrated up to this dose after a week or so if they have been tolerating the 25 mg dose and have persistent symptoms.
Patients who have persistent symptoms or the symptoms have not improved by 50% after two to four weeks can increase the dose to 100 mg twice daily. Patients with advanced kidney disease such as those with a CrCl of less than 30 ml/minute should be advised not to exceed a dose of 50 mg twice daily.
Milnacipran (Ixel) Side effects and Safety Concerns:
Milnacipran Safety concerns:
- Renal Impairment:
- Milnacipran should not be used in patients with end stage kidney disease. In patients with moderate to severe renal impairment such as those with a CrCl of less than 30 ml/minute should not exceed a dose of 50 mg twice daily.
- Liver disease:
- It should not be given to patients who have moderate to severe hepatic impairment. Cases of acute liver failure, drug-induced hepatitis, and raised liver enzymes have been reported with the use of milnacipran.
- Glaucoma:
- It should also be avoided in patients with angle closing glaucoma and those who may be at risk of developing acute-angle closure glaucoma.
Milnacipran side effects:
Side effects of the drug can be one of the major cause of treatment discontinuation. Most of the drug side effects are related to the gastrointestinal system or the neurological system.
The incidence of side effects increases when the dose is increased. With higher doses and especially at doses exceeding 50 mg twice daily, the frequency of side effects is much more than at low doses.
Furthermore, side effects are more frequent during the first few weeks of treatment initiation. Patients who continue to use the drug beyond three months have little drug-related adverse reactions.
The following are some of the most common side effects of milnacipran:
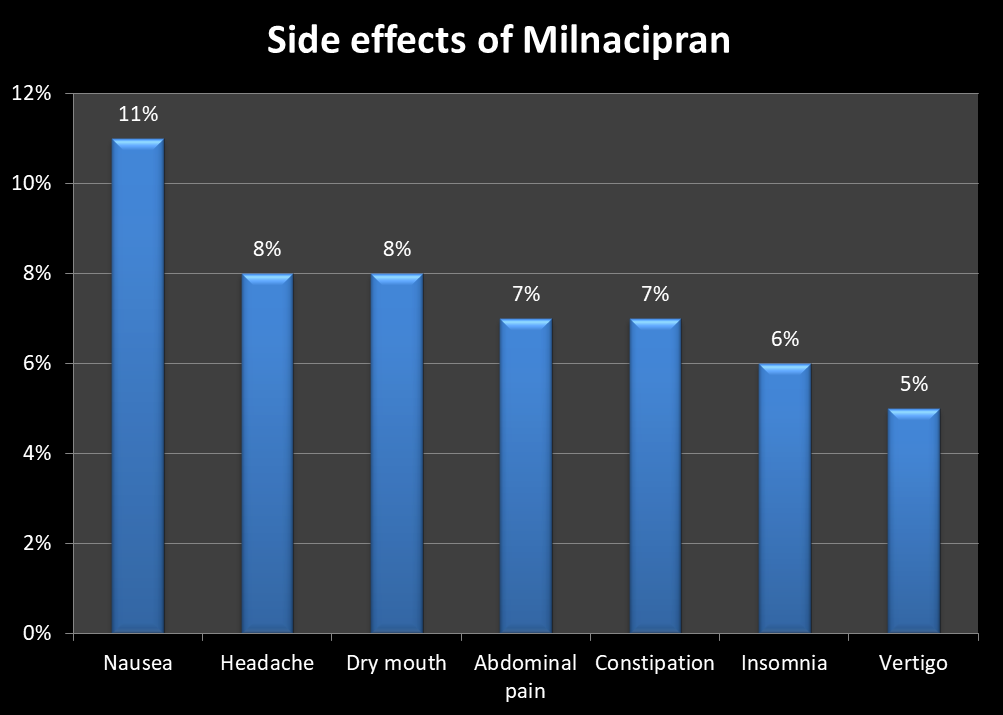
It is important to monitor the blood pressure. Like all other SNRIs, milnacipran can also result in an increase in the blood pressure. Individuals with hypertension or pre-hypertension should monitor their blood pressure and adjust their dose accordingly. Blood pressure should be monitored periodically every two to six months thereafter.
A rise of 1 to 3 mmHg of blood pressure has been observed in previously healthy individuals. Those with pre-existing hypertension, a rise in the blood pressure of up to 15 mmHg has been observed.
How to Discontinue Milnacipran (Ixel)?
Like all antidepressants, it is recommended to slowly taper off Milnacipran. Abrupt discontinuation can result in withdrawal symptoms. Withdrawal symptoms can appear as tremors, irritability, anxiety, depression, aggression, insomnia or somnolence, and gastrointestinal symptoms like nausea and vomiting.
To discontinue the dose is reduced to half and continued for about two weeks. If the patient has been on high doses such as 100 mg twice daily for a long time, the dose can be halved and continued for two to four weeks and then halved to 12.5 mg for another two to four weeks. If the patient is tolerating the low dose, he/she can either discontinue it or take it for a week or so on alternate days before totally discontinuing it.
Read: Levomilnacipran (Brand Name Fetzima), Uses, Dosage, Side effects, MOA