Januvia is the brand name of Sitagliptin. It is a prescription medication used to treat Type 2 Diabetes Mellitus. Januvia can be taken alone or in combination with other medications.
Januvia is a member of the Dipeptidyl Peptidase IV Inhibitors class of drugs. Other drugs in the same group include:
What is Januvia (Sitagliptin)
JANUVIA tablets contain sitagliptin phosphate which is an orally active inhibitor for the dipeptidyl peptidase 4 (DPP-4) enzyme.
Each film-coated tablet from JANUVIA contains 32.13 to 64.25 or 128.5 mg sitagliptin phosphate monohydrate. This is equivalent to 25, 50, or 100 mg of the free base, as well as the following inactive ingredients; microcrystalline cellulose and anhydrous dibasic Calcium phosphate, croscarmellose, magnesium stearate, and sodium stearyl fumarate. The film coating also contains the inactive ingredients polyvinyl alcohol and polyethylene glycol.
Januvia (Dosage & Indications)
JANUVIA Indications:
JANUVIA (Sitagliptin), is used to supplement diet and exercise in order to improve glycemic control for adults with type 2 diabetes mellitus.
Limitations of Januvia Use:
JANUVIA should be avoided in patients with type 1 diabetes or in the treatment of diabetic ketoacidosis.
Patients with a history or present symptoms of pancreatitis have not been treated with JANUVIA. Patients with a history or present symptoms of pancreatitis may be at greater risk of developing it while taking JANUVIA.
It should be preferably avoided in patients with a history of pancreatitis
Januvia (Dosage and Administration)
Dosage Recommendations:
- 100mg of JANUVIA should be taken once daily. JANUVIA can be taken with or without food.
Januvia Dosage Recommendations for Renal Impairment:
- Patients with an estimated glomerular filtration rate [eGFR] of 45 mL/min/1.73 m² or more can take it without any dosage adjustments.
- Patients with moderate renal impairment (eGFR greater or equal to 30 mL/min/1.73 m² or less than 45 mL/min/1.73 m²) should take a maximum dose of 50 mg of JANUVIA once daily.
- Patients with severe renal impairment (eGFR below 30 mL/min/1.73 m²) and patients with end-stage kidney disease (ESRD), who require hemodialysis (or peritoneal) or hemodialysis, can take a maximum dose of 25 mg of JANUVIA once daily. JANUVIA can be given at any time, regardless of the timings of dialysis.
Renal functions should be monitored before and after treatment initiation. Postmarketing reports have shown that patients with impaired renal function were experiencing worsening, and some were given inappropriate sitagliptin doses.
Januvia Dosage Forms and Strengths
- 100 mg tablets are round, beige tablets coated with film and containing “277” on the one side.
- 50 mg tablets are round, light-beige, film-coated tablets that have “112” printed on one side.
- 25 mg tablets are round, pink, film-coated tablets containing “221” on one side.
How to Store Januvia?
Keep your room at 20-25°C (68-77°F), and allow for excursions to 15-30°C (59-86°F).
Januvia (Sitagliptin) Side Effects
Clinical Trials Experience:
The overall incidence of adverse reactions and hypoglycemia in controlled clinical trials with Sitagliptin, whether monotherapy or combination therapy, was similar to placebo. Combining Sitagliptin with glimepiride with or without metformin resulted in a higher rate of clinical adverse reactions than with placebo. This was partly due to a higher incidence of hypoglycemia. The incidence of discontinuation because of clinical adverse reactions was comparable to placebo.
During Week 54 of the study of JANUVIA (combination therapy with metformin and rosiglitazone) adverse reactions were reported regardless of the investigator’s assessment of causality. These adverse reactions, which occurred more often than patients who received placebo, and in greater numbers than those who received Sitagliptin, included:
- Upper respiratory tract infection (JANUVIA; 15.5%; placebo (6.2%)),
- nasopharyngitis (11.1%), 9.3%),
- peripheral edema (5.3, 5.2%),
- headache (5.5%, 4.1%).

A pooled analysis of two monotherapy studies, one to add-on metformin and one to pioglitazone, revealed that certain gastrointestinal adverse reactions were more common in patients receiving JANUVIA 100mg.
A 24-week study with JANUVIA and pioglitazone as initial therapy revealed no adverse reactions in more than 5% of patients. This is in contrast to patients who received pioglitazone only.
Patients treated with JANUVIA did not experience any clinically significant changes in vital signs, ECG (including QTc interval), or ECG.
Side effects of Januvia: Hypoglycemia
Hypoglycemia is the most common adverse drug reaction. This is especially true when Januvia is combined with other diabetes medications such as Insulin or sulfonylurea. Patients who received Sitagliptin with insulin or a sulfonylurea had a higher incidence of hypoglycemia than those who were given the placebo.
A pooled analysis of two monotherapy studies, one with add-ons to metformin and one with add-ons to pioglitazone, showed that the incidence of adverse hypoglycemia reactions was 1.2% for patients who received Sitagliptin 100mg and 0.9% for patients who received placebo.
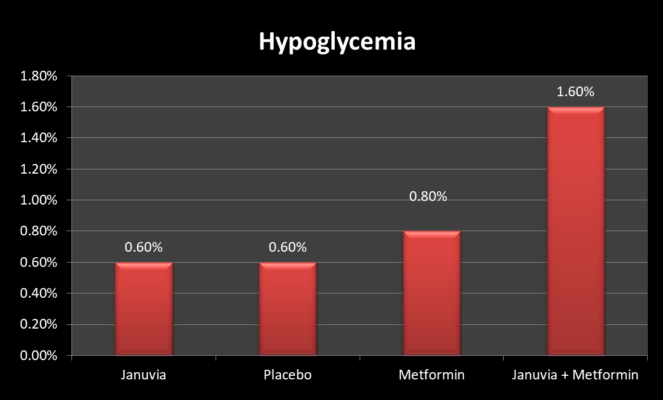
The 24-week placebo-controlled factorial study on initial treatment with Sitagliptin and metformin combined showed that hypoglycemia occurred in 0.6% of patients who received placebo, 0.6% of patients who received Sitagliptin only, 0.8% of patients who received metformin alone, and 1.6% of patients who received Sitagliptin with metformin.
Effect of Januvia on Laboratory Tests
Clinical studies showed that patients who received JANUVIA 100mg had a similar rate of adverse reactions to placebo.
- An increase in neutrophils was responsible for a small increase in white blood cells count (WBC). The increase in WBC of 200 cells/microL vs placebo in four placebo-controlled clinical trials with a mean baseline WBC count at 6600 cells/microL is not clinically relevant.
- A 12-week study of 91 patients suffering from chronic renal disease included 37 patients with moderate renal impairment who were randomized daily to JANUVIA 50 mg and 14 patients with similar levels of impairment. Patients treated with JANUVIA experienced a mean increase in serum creatinine of 0.12 mg/dL (0.04), compared to patients who received placebo (0.07 mg/dL (0.07). It is unknown what the clinical significance of this increased serum creatinine relative placebo.
Additional Side effects of Januvia (Sitagliptin):
- Anaphylaxis, urticaria, and exfoliative conditions such as Stevens-Johnson syndrome
- Hepatic enzyme elevations and acute Pancreatitis including fatal and non-fatal hemorrhagic and necrotizing Pancreatitis
- Severe and disabling arthritis
- Bullous Pemphigoid and stomatitis,
- Rhabdomyolysis
Januvia (Sitagliptin) Drug Interactions:
Januvia Interactions with Digoxin
With the coadministration of 100mg sitagliptin over a period of 10 days, there was a slight rise in the area below the curve (AUC) and mean peak drug concentrations (Cmax) of digoxin. Digoxin patients should be closely monitored. It is not recommended to adjust the dosage of digoxin and JANUVIA.
Januvia use in combination with Insulin Secretagogues Or Insulin
JANUVIA may be co-administrated with an insulin secretagogue (e.g. sulfonylurea or insulin), however, the risk of hypoglycemia is increased and should be monitored.
Warnings and Precautions When using Januvia (Sitagliptin)
Januvia and the risk of Pancreatitis:
- Postmarketing reports have shown that patients who took JANUVIA suffered from acute pancreatitis. This includes fatal or non-fatal hemorrhagic and necrotizing pancreatitis.
- Patients should be closely monitored for symptoms and signs of pancreatitis after JANUVIA is started.
- If you suspect that you have pancreatitis, JANUVIA should immediately be stopped and the appropriate management started. It is not known if patients who have had pancreatitis in the past are more likely to develop it while taking JANUVIA.
Januvia and the Risk of Heart Failure:
Cardiovascular outcomes trials of two other DPP-4 inhibitors showed a link between heart failure and dipeptidyl peptidase-4 treatment. These trials evaluated patients suffering from type 2 diabetes and atherosclerotic heart disease.
Before initiating treatment for patients at high risk of developing heart disease, such as those who have had heart attacks or are suffering from renal impairment, it is important to consider the risks and benefits associated with JANUVIA.
During therapy, be sure to monitor these patients for any signs and symptoms that could indicate heart failure. Inform patients about the symptoms of heart disease and urge them to report any such symptoms immediately. Assess and manage heart failure according to current care standards and discontinue JANUVIA.
Januvia and Renal dysfunction:
Before starting JANUVIA, and every other day thereafter, it is advisable to assess your renal function. Patients with severe or moderate renal impairment, patients who require hemodialysis or peritoneal dialysis should be adjusted in dosage. Patients with severe (eGFR 30 mL/min/1.73 m² or less) renal impairment should be cautiously prescribed JANUVIA.
Postmarketing reports have indicated that patients with chronic renal disease, such as acute renal failure, may experience worsening renal function. One subset of these cases involved patients with severe renal impairment who were given inappropriate sitagliptin doses.
With supportive treatment, discontinuation of potentially causative drugs, and supportive treatment, a return to baseline levels has been observed. If another etiology is suspected to have caused the acute decline in renal function, it may be worth cautiously reinitiating JANUVIA.
Preclinical studies of JANUVIA at clinically relevant doses and in clinical trials have not shown JANUVIA to be nephrotoxic.
Januvia Use with other hypoglycemic medications:
JANUVIA, when taken with a sulfonylurea, or insulin, which are known to cause hypoglycemia in patients, caused an increase in the incidence of hypoglycemia. To reduce hypoglycemia, it may be necessary to take a lower dose of insulin.
Januvia and Hypersensitivity Reactions:
Postmarketing reports have shown that patients who were treated with JANUVIA experienced severe hypersensitivity reactions. These reactions include anaphylaxis and angioedema as well as exfoliative skin conditions such as Stevens-Johnson syndrome. These reactions usually occur within 3 months of JANUVIA treatment. Some reports have occurred after only one dose. If you suspect that there is a hypersensitivity reaction, stop taking JANUVIA and consult your doctor to rule out other possible causes.
Other DPP-4 inhibitors have also been linked to angioedema. Patients who have had angioedema from another DPP-4 inhibitor should be cautious as it is not known if they will be more susceptible to angioedema when taking JANUVIA.
Januvia and severe disabling Arthralgias:
Postmarketing reports have shown that patients receiving DPP-4 inhibitors can suffer from severe and disabling arthritis. From one day to several years, the time it took for symptoms to manifest after initiation of medication therapy could vary. Patients felt relief after discontinuing the medication. Some patients experienced recurrences of their symptoms after restarting the drug or another DPP-4 inhibitor. DPP-4 inhibitors may be a cause of severe joint pain. If this is the case, discontinue using drugs.
Januvia and Bullous Pemphigoid:
DPP4 inhibitors have been used in postmarketing cases of bullous pemphigoid that required hospitalization. Patients generally recovered after receiving topical or systemic immunosuppressive therapy and ceasing to take the DPP-4 inhibitor. Inform patients to immediately report any blisters or other skin problems while they are receiving JANUVIA. Bullous pemphigoid should be suspected and JANUVIA should not be continued. Refer to a dermatologist for appropriate treatment and diagnosis.
Januvia and Macrovascular Outcomes:
No clinical studies have shown that JANUVIA reduces macrovascular risk.
Patient Counselling regarding the side effects of Januvia:
Pancreatitis:
- Inform patients that acute pancreatitis was reported after JANUVIA was approved for post-marketing use. Acute pancreatitis is characterized by persistent severe abdominal pain that radiates to the back and maybe accompanied or not by vomiting. If severe abdominal pain persists, instruct patients to stop taking JANUVIA immediately and consult their doctor.
Heart Failure:
- Tell patients about the symptoms and signs of heart disease. Ask patients about their history of heart disease or other risk factors, such as moderate to severe renal impairment. If patients experience symptoms such as shortness of breath, weight gain, or swelling, they should contact their doctor immediately.
Hypoglycemia:
- Tell patients that JANUVIA can increase the likelihood of hypoglycemia when it is combined with sulfonylurea/insulin. A lower dose of the sulfonylurea/insulin may be necessary to reduce the chance of hypoglycemia.
Hypersensitivity Reactions:
- Inform patients that allergic reactions were reported after Sitagliptin was approved for marketing. Patients should immediately seek medical advice if they experience symptoms such as hives, rash, or swelling of their throats, which could cause difficulty breathing or swallowing.
Arthralgia that is severe and disabling:
- This class of drugs can cause severe and debilitating joint pain. It can take anywhere from one to three years for symptoms to manifest. If severe pain is experienced in the joints, inform patients to consult a physician.
Bullous Pemphigoid:
- Patients should be aware that this drug class may cause bullous pemphigoid. Inform patients to seek medical attention if they develop blisters or erosions.
Januvia Use in Specific Populations:
Januvia use in Pregnancy:
There is not enough data on sitagliptin use in pregnancy to determine if there is a risk of miscarriage or major birth defects. Poorly controlled diabetes during pregnancy poses risks for the mother and baby. Sitagliptin administration to pregnant rabbits and rats during organogenesis was without any adverse developmental effects. The oral doses were up to 30-times or 20-times the clinical dose of 100 mg, based upon AUC.
Pre-gestational diabetic women who have a Hemoglobin A1c greater than 7% are at risk for major birth defects. However, it has been reported that the background risk is as high as 20% to 25% for women with Hemoglobin A1c above 10%. The background risk of miscarriage and major birth defects in pregnancies that are clinically diagnosed in the United States is 24% and 15%, respectively.
Poorly managed diabetes during pregnancy increases the maternal risk of diabetic ketoacidosis and preeclampsia. Preterm births are more common than spontaneous abortions. Poorly managed diabetes can increase the risk of major birth defects, stillbirth, and other complications.
Januvia Use during Breastfeeding:
It is not known whether JANUVIA is present in human milk or whether it has any effects on breastfed infants. Sitagliptin can be found in human milk, as well as in rat milk. Consider the developmental and health benefits of breastfeeding, as well as the maternal clinical need for sitagliptin. Also, consider any adverse effects that sitagliptin may have on the infant or the underlying condition.
Januvia Use in children:
Pediatric patients below 18 years of age should not take sitagliptin as the safety and efficacy in younger age groups have not been proven.
Januvia Use in Older patients:
725 of the pre-approval clinical safety & efficacy studies of JANUVIA included subjects over 65 years old, and 61 were over 75 years. There were no differences in safety and effectiveness between subjects 65 and over and those under 65. This and other clinical experiences have not shown any differences in the responses of older patients and younger patients. However, it is possible that some older people are more sensitive.
Sitagliptin is substantially excreted by your kidneys. Because of this, it should be evaluated more often in elderly patients.
Januvia use in Renal Impairment:
Sitagliptin is excreted by kidneys. Patients with impaired renal function may be exposed to sitagliptin at higher doses. Patients with eGFR below 45 mL/min/1.73 m² (moderate or severe renal impairment) and patients requiring dialysis are advised to take lower doses.
Januvia Overdosage and Contraindications
DO NOT OVERDOSE
Contact the Poison Control Center if you have taken sitagliptin overdose.
It is possible to use supportive measures in the event of an overdose. These include removing unabsorbed material from your gastrointestinal tract, using clinical monitoring (including an electrocardiogram), and instituting supportive therapy according to the patient’s clinical condition.
Sitagliptin is moderately dialyzable. Clinical studies showed that approximately 13.5% of the dose was eliminated in a three- to four-hour hemodialysis session. If clinically necessary, prolonged hemodialysis might be considered. Peritoneal dialysis may be able to dialyze sitagliptin.
Read: Rybelsus vs Januvia and Janumet
Januvia (Sitagliptin) Pharmacology:
Januvia MOA (Mechanism of Action):
Sitagliptin, a DPP-4 inhibitor, is thought to be responsible for the actions it exerts in type 2 diabetes mellitus patients by slowing down incretin hormone inactivation. Sitagliptin increases the concentrations of active intact hormones, which in turn prolongs and enhances the action of these hormones.
The intestine releases various hormones throughout the day including glucose-dependent insulinotropic peptide (GIP) and glucagon-like Peptide-1 (GLP-1). These hormones are also increased by eating.
DPP-4 quickly activates these hormones. Incretins are part an endogenous system that regulates glucose homeostasis. GLP-1 or GIP are intracellular signaling pathways that involve cyclicAMP and increase insulin synthesis from pancreatic beta cells when blood glucose levels are normal or high.
GLP-1 also reduces pancreatic alpha cell glucagon secretion, which results in decreased hepatic glucose production. Sitagliptin increases insulin production and decreases blood glucose levels by prolonging and increasing active incretin levels. Sitagliptin is selective for DPP-4, and it does not inhibit DPP-8/DPP-9 activity in vitro at concentrations that are similar to therapeutic doses.
Sitagliptin was administered to patients suffering from type 2 diabetes mellitus. This resulted in the inhibition of DPP-4 enzyme activity over a 24-hour period. This DPP-4 inhibition caused a 2-to-3-fold increase in active GLP-1 levels and GIP in the blood, decreased glucose concentrations and increased insulin release to glucose. A decrease in glucose and a rise in insulin was associated with lower fasting glucose levels.
Sitagliptin was not found to cause hypoglycemia or blood glucose drops in healthy subjects.
Sitagliptin And Metformin Hydrochloride Coadministration
- Sitagliptin and metformin both increased active GLP-1 levels in healthy subjects over a 2-day period. Metformin alone raised total and active GLP-1 levels to comparable degrees. Sitagliptin and metformin coadministration had an additive effect upon active GLP-1 levels. Sitagliptin but not metformin increased active GIP levels. These findings are not related to changes in glycemic control for patients with type 2 diabetes mellitus.
Sitagliptin (Januvia) effect on Cardiac Electrophysiology:
- A placebo-controlled, randomized crossover study was conducted in which 79 healthy subjects received sitagliptin 100mg, sitagliptin 800-mg (8 times the recommended dose), as well as placebo.
- The recommended dose of 100mg had no effect on the QTc interval at peak plasma concentration or any other time in the study. The maximum placebo-corrected increase in QTc was observed after 800 mg and it was 8.0msec. This is not clinically significant. Peak sitagliptin plasma levels at 800 mg were approximately 11 times greater than those following 100-mg.
- Patients with type 2 diabetes mellitus were given sitagliptin 100mg (N=81), sitagliptin 20mg (N=63) each day. There were no significant changes in QTc intervals based on ECG data at the expected peak plasma concentration.
Januvia Pharmacokinetics:
Sitagliptin’s pharmacokinetics have been extensively studied in healthy subjects as well as patients suffering from type 2 diabetes mellitus. After a single 100-mg oral dose, sitagliptin’s plasma AUC was 8.52 mM*hr. Cmax was 950nM.
The apparent terminal half-life (t1/2), was 12.4 hours. The plasma AUC of sitagliptin rose in proportion to the dose and was approximately 14% higher after 100 mg doses at constant-state than it was following the initial dose.
Sitagliptin’s AUC had small intra- and intersubject coefficients. They were 5.8% and 15.1%, respectively. Sitagliptin’s pharmacokinetics were generally the same in healthy subjects as in patients with type II diabetes mellitus.
Januvia Absorption:
Sitagliptin was quickly absorbed by healthy subjects after being administered orally in a 100mg dose. Peak plasma concentrations (median Tmax), occurred within 1 to 4 hours. Sitagliptin’s absolute bioavailability is 87%.
The Effect of Food
The pharmacokinetics and safety of sitagliptin were not affected by sitagliptin coadministration of high-fat meals.
Distribution
Sitagliptin intravenous 100-mg dose to healthy subjects results in a mean volume of distribution of approximately 198 liters. Low (38%) is the percentage of sitagliptin that is reversibly bound with plasma proteins.
Elimination of Sitagliptin:
Sitagliptin excreted unchanged in urine is approximately 79%. Metabolism is a minor route of elimination. After taking 100 mg sitagliptin orally, the apparent terminal t1/2 was approximately 12.4 hours. Renal clearance was approximately 350mL/min.
Metabolism of Januvia:
After taking sitagliptin orally, approximately 16% radioactivity was removed as sitagliptin metabolites. Six metabolites of sitagliptin were detected in trace amounts. They are unlikely to be responsible for the plasma DPP-4 inhibitory effect. In vitro studies showed that sitagliptin’s limited metabolism was primarily caused by CYP3A4, with a contribution from CYP2C8.
Excretion of Januvia:
After administration of an oral sitagliptin dosage to healthy subjects, approximately 100 percent of the administered radioactivity was eliminated in feces (13%) and urine (87%) within a week.
Sitagliptin is primarily eliminated by renal excretion, and active tubular secretion. Sitagliptin may play a role in renal sitagliptin elimination by being a substrate for the human organic anion transporter-3. It is not known if hOAT-3 has any clinical significance in sitagliptin transportation. Sitagliptin may also be a substrate for P-glycoprotein, which could also play a role in the renal elimination. Cyclosporine, a Pgp inhibitor, did however not decrease sitagliptin’s renal clearance.
What information is most important about JANUVIA and what should I know?
Side effects of JANUVIA can be serious, such as:
Pancreatitis:
- Pancreatitis is an inflammation of the pancreas that can lead to severe complications and even death. You are more likely to develop pancreatitis if you have certain medical conditions.
Tell your doctor if you have had any previous JANUVIA before you begin taking it.- Pancreatitis
- High blood triglyceride levels
- Gallstones are stones found in the gallbladder.
- kidney problems
- A history of alcoholism
- If you feel pain in your abdomen (abdomen), stop taking JANUVIA immediately and contact your doctor. You may feel the pain radiating from your stomach to your back. It may be accompanied or unaccompanied by vomiting. These could be signs of pancreatitis.
Heart failure:
- Heart failure is when your heart stops pumping blood efficiently enough. Tell your doctor if your history includes heart disease or kidney problems before you begin taking JANUVIA.
- Especially when you are lying down, your difficulty breathing or shortness of breath can increase.
- Fluid retention or swelling, particularly in the feet, ankles, or legs
- A remarkablely rapid weight gain
- Unusual tiredness
- These could be signs of heart disease.
What are the side effects of JANUVIA?
People who have taken JANUVIA have experienced serious side effects.
Hypoglycemia:
- Hypoglycemia is low blood sugar. Your risk of developing low blood sugar if you take JANUVIA along with a sulfonylurea, insulin, or other medicine that can cause it is greater. JANUVIA may require you to lower the dose of your sulfonylurea medication or insulin. Low blood sugar can cause:
- Drowsiness
- irritability
- hunger
- dizziness
- There is confusion, sweating, jitteriness, and weakness
- Fast heartbeat
- Severe allergic reactions.
Allergic reactions:
- You should immediately stop using JANUVIA if you experience symptoms that could indicate a serious allergic reaction. Your doctor might prescribe another medicine to treat your diabetes or give you an allergy medicine.
Joint pains:
- Joint pain can sometimes be severe for people who are taking DPP-4 inhibitors, such as JANUVIA. If you experience severe joint pain, consult your doctor.
Skin reactions:
- Bullous pemphigoid is a skin reaction that may occur in people who are taking DPP-4 inhibitors such as JANUVIA. This can lead to needing treatment at a hospital. If you experience blisters or the loss of skin’s outer layer (erosion), tell your doctor immediately. Your doctor might tell you to stop using JANUVIA.
Respiratory Infections:
- The most common side effects are upper respiratory infection, stuffy nose, sore throat, headache, and runny nose.
Stomach Upset:
- JANUVIA can cause stomach upset, diarrhea, and swelling of hands and feet when taken with rosiglitazone (Avandia).
Read: Sitagliptin vs Vildagliptin: Comparing Januvia Vs Galvus
