Xarelto (Rivaroxaban) is a factor Xa inhibitor. It is used to treat and prevent blood clots. In comparison to warfarin, it does not require weekly monitoring of INR. It is also less likely to cause major bleeding such as intracranial bleeding.
Xarelto Dosage Forms and Strengths:
It is available as a Tablets formulation, administered orally once or twice a day.
- 2.5mg
- 10mg
- 15mg
- 20mg
Xarelto Indications (Uses):
Xarelto (Rivaroxaban) is used to treat and prevent blood clots in the blood vessels. It is also being used in the prevention of blood clots in patients with COVID-19 infection.
Xarelto Dose for Deep Vein Thrombosis Prophylaxis to Prevent Hip and Knee Replacement Surgery
- Prophylaxis for deep vein thrombosis (DVT) is indicated. This could lead to pulmonary embolism in patients who have had knee or hip replacement surgery.
- After hemostasis is established, you can start 6-10 hours after the surgery.
- Replacement of the knee: 10mg PO once a day for 12 Days
- Hip replacement: 10mg PO once a day for 35 Days
Xarelto for Prophylaxis for Venous Thromboembolism and Restricted Mobility:
- Prophylactic treatment of venous embolism (VTE), and VTE-related deaths during hospitalization and discharge in acutely ill patients admitted to the hospital and who are at risk of developing thromboembolic complications.
- 10 mg orally daily, in the hospital or after discharge from hospital, for 31-39 Days
Xarelto for DVT and/or treatment with pulmonary embolism:
- This is indicated for the treatment of DVT or PE.
- 15 mg orally every 12 hourly for 21 days, then 20 mg orally once a day
Reduced Stroke Risk in Nonvalvular Arial Fibrillation
- Patients with nonvalvular atrial fibrillation are recommended to lower the risk of strokes and systemic embolism.
- 20 mg orally once a day.
Xarelto to reduce the risk of recurrence of DVT/PE
- Patients at increased risk of recurrent DVT or PE following treatment for at least six months are recommended to reduce their risk of it happening again.
- After at least 6 months of anticoagulant treatment, 10 mg orally once daily.
Xarelto dose for the reduction of Major Cardiovascular Events
- Combination of aspirin and cardiovascular risk reduction in patients with chronic coronary disease (CAD).
- 2.5 mg orally twice daily, plus aspirin (75-100 mg) daily.
Xarelto for the reduction of Risk of Major Thrombotic Vascular Events
A combination of aspirin and Xarelto is indicated to lower the risk of major strokes, ischemic strokes, and major amputations of a vascular cause in patients with the peripheral arterial disease (PAD). This includes those who have had a lower extremity procedure to treat symptomatic PAD.
- 2.5 mg orally twice daily, plus aspirin (75-100 mg) per orally once daily.
- After a successful lower extremity revascularization procedure, initiate therapy once hemostasis is established.
Xarelto Dosage modifications
Use in combination with Strong CYP3A4 Inhibitors and Inducers (P-gp)
- Use caution when co-administration is done with strong CYP3A inhibitors and combined P-gp.
- Use caution when co-administration is done with strong CYP3A inducers and combined P-gp.
Renal impairment (risk reduction for major cardiovascular events).
- No dose adjustment is required in patients with mild renal impairment.
- ESRD patients not undergoing dialysis.
- 2.5 mg twice daily will give an exposure comparable to that of patients with moderate renal impairment. Patients with preserved renal function had similar safety and efficacy outcomes to those with moderate renal impairment.
- ESRD on dialysis:
- There are no clinical outcomes data for aspirin use in patients with ESRD who are on intermittent hemodialysis. 2.5mg BID was administered to patients with ESRD. The concentrations and pharmacodynamic activities were similar to those seen in moderately renal impaired patients in COMPASS.
Reduction of the risk of recurring DVT/PE, prophylaxis for DVT, prophylaxis for VTE in patients with renal impairment:
- CrCl >=30 mL/min:
- No dosage adjustment is necessary
- CrCl 15-30 mL/min:
- There are limited clinical data. Monitor closely and evaluate any signs and symptoms of blood loss.
- CrCl 15 mL/min:
- ESRD on dialysis
- Patients who experience acute renal failure in treatment should be discontinued
Treatment Nonvalvular Atrial fibrillation in patients with renal impairment:
- CrCl >50 mL/min:
- No dosage adjustment is necessary
- CrCl 15-50 mL/min:
- 15 mg PO qDay
- ESRD for intermittent renal dialysis:
- 15mg/day
Xarelto dose in patients with hepatic impairment:
- Moderate (Child Pugh B):
- It has been observed that the AUC increases by 127%; we recommend that you not use them
- Severe (Child Pug B or C) or any other hepatic disease that is associated with coagulopathy should be avoided. There are no clinical data.
Dosing considerations before a surgical procedure:
For surgery or other procedures, discontinue
- At least 24 hours before the procedure, stop taking rivaroxaban
- After surgery or procedure, you can resume rivaroxaban as soon as sufficient hemostasis has been established
- Consider administering a parenteral medication to someone who is unable or unwilling to take an oral medication after undergoing surgery.
Switching to Rivaroxaban from other blood thinners:
- From warfarin to rivaroxaban:
- Discontinue the use of warfarin and begin rivaroxaban as soon as INR is close to 3.
- To switch from anticoagulant other than warfarin to rivaroxaban (eg, low-molecular-weight heparin):
- You must start rivaroxaban between 0 – 2 hours before the next evening’s administration. You can also omit the administrations of other anticoagulants.
- Unfractionated Heparin continuous Infusion to Rivaroxaban:
- Stop infusion and begin rivaroxaban simultaneously
Moving from rivaroxaban to other blood thinners:
- From rivaroxaban to warfarin:
- There are no clinical data; INR measurements taken while co-administration with warfarin might not be helpful in determining the appropriate dose of warfarin. One approach is to stop taking rivaroxaban altogether and to start both a parenteral and oral anticoagulant at the time of the next scheduled dose.
- From rivaroxaban to another rapid-onset anticoagulant:
- Stop taking rivaroxaban. Give the first dose of another anticoagulant (PO/parenteral) at your next scheduled rivaroxaban dosage
Side Effects of Xarelto (rivaroxaban):
Relatively frequent side effects (1-10%):

Major bleeding as a side effect of Xarelto (Rivaroxaban):
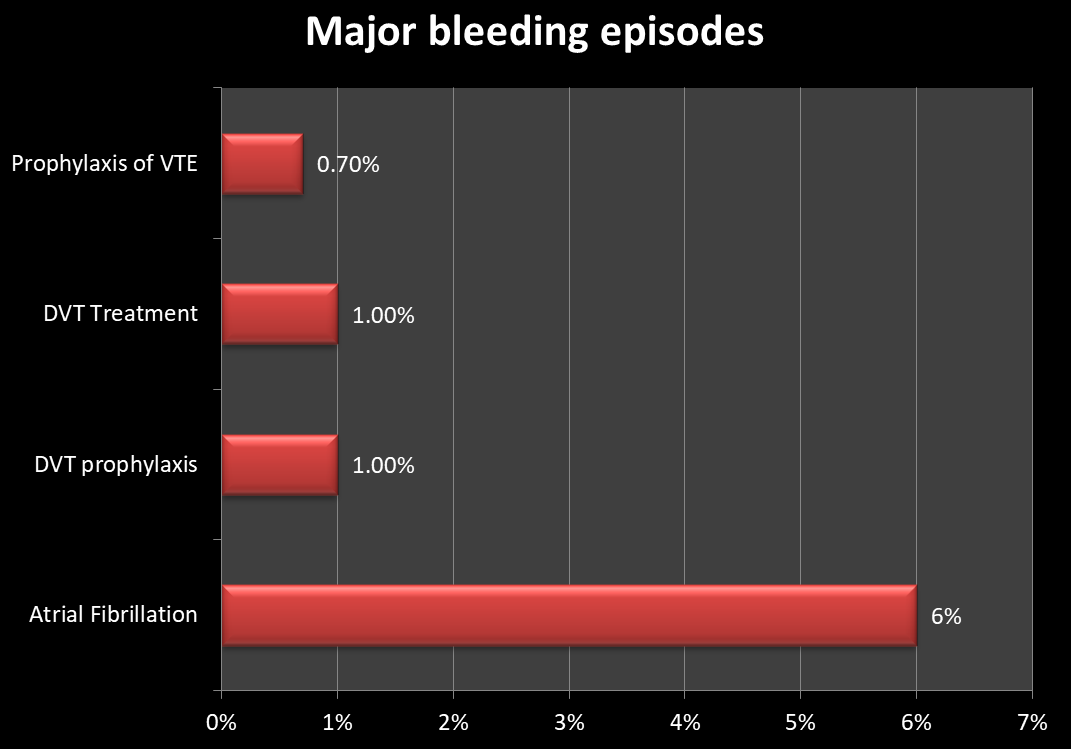
Post-marketing reports
Disorders of the blood and lymphatic systems:
- Agranulocytosis and Thrombocytopenia
Gastrointestinal Disorders:
- Retroperitoneal Hemorrhage
Hepatobiliary conditions:
- Cholestasis, Jaundice, and Hepatitis (including hepatocellular Injury)
Immune system disorders:
- Hypersensitivity, anaphylactic reaction, anaphylactic shock, and angioedema
Nervous system disorders:
- Cerebral hemorrhage, subdural and epidural hematomas, and hemiparesis.
Skin and SC tissue disorders:
- Stevens-Johnson syndrome, and drug reaction with the eosinophilia (DRESS),
Xarelto Warnings
Spinal hematomas or epidural hematomas
Patients who are anticoagulated, receiving neuraxial or spinal puncture may experience this. It is important to consider the risks and benefits of neuraxial interventions for patients who are not anticoagulated. The optimal timing between therapy administrations and neuraxial procedures is unknown.
These hematomas can cause permanent or long-term paralysis. Be aware of these risks when scheduling patients to undergo spinal procedures
Factors that increase risk include:
- Co-administration with drugs that affect hemostasis such as non-steroidal anti-Inflammatory Drugs (NSAIDs),
- Co-administration of epidural catheters in the body,
- History of epidural punctures or spinal punctures,
- History of spinal deformity, or surgery.
You should be on the lookout for any signs or symptoms of neurologic impairment. If you notice any, immediate treatment is required.
The risk of developing thrombotic complications increases if you discontinue treatment too soon
Patients are at greater risk of thrombotic events if they stop taking anticoagulants such as rivaroxaban before the prescribed time.
Consider switching to another anticoagulant if anticoagulation with Rivaroxaban has to be stopped for any reason other than bleeding from the brain.
Contraindications to Xarelto (Rivaroxaban):
- Hypersensitivity
- Active pathological bleeding
- Transcatheter aortic replacements (Tavr)
Cautions and Warnings:
Neuraxial Anesthesia (Black Box Warning):
See Warnings above.
Premature Discontinuation:
- Premature discontinuation can increase the risk of thrombotic events (see Black Box Warnings).
Prosthetic Valves:
- Patients with prosthetic hearts valves have not had safety and efficacy studies; they are not recommended for patients who use them.
Bleeding:
- This increases the risk of bleeding and can lead to serious and fatal bleeding. There are reports of major hemorrhages including adrenal bleeding, epidural hemorrhages, and intracranial hemorrhages. It is important that you promptly assess the signs and symptoms of blood losses and determine if blood replacement is necessary; stop taking active pathological hemorrhages.
Patients requiring primary VTE prophylaxis are not recommended to use this medication if they have:
- history of bronchiectasis or pulmonary cavitation;
- active cancer (i.e. Patients undergoing acute, in hospital cancer treatment.
- History of bleeding in the 3 months preceding treatment.
Unstable patients:
- Patients with PE with hemodynamic instability, thrombolysis, or pulmonary embolectomy are not recommended to use Xarelto. Unfractionated heparin is an acute alternative to it.
Catheter removal:
- Never remove an intravenous epidural catheter or intrathecal catheter prior to at least 2 half-lives (ie. 18 hours for patients aged 20-45 and 26 hours for patients aged 60-76), following the last administration. Do not give the next dose any earlier than 6 hours after catheter removal; if there is a traumatic puncture, wait 24 hours before administering.
Impaired Renal Functions:
Assess renal function periodically as clinically indicated (i.e. more often in situations where renal function may decline). Adjust therapy accordingly. Consider dose adjustment or discontinuation for patients with acute renal failure.
Thrombosis in patients with APS (antiphospholipid syndrome):
- Patients with a history of thrombosis due to APS should not be treated with Xarelto (Rivaroxaban). Treatment of patients with APS, especially those who are triple-positive (positive for anticardiolipin, lupus anticoagulant, and anti-beta 2 glycoprotein 1 antibodies) has been shown to increase the likelihood of recurrent events than vitamin K antagonist therapy.
Pregnancy:
- Be cautious with pregnant women. Only use if there is a clear benefit to the mother and the fetus. (See Pregnancy).
How to reverse the effects of Xarelto (Rivaroxaban):
Procoagulant reversal agents such as prothrombin concentrate (PCC), activated prothrombin compound concentrate (APC), or recombinant factors VIIa may be used when anticoagulation needs to be reversed due to life-threatening bleeding or uncontrolled bleeding.
Andexanate is an antidote of Rivaroxaban. It is indicated for the treatment of life-threatening bleeding in patients taking Xarelto.
Important drug interactions of Xarelto (Rivaroxaban):
Rivaroxaban can be used as a substrate for CYP3A4/5 and CYP2J2, as well the P-gp (ABCG2) and ATP binding cassette G2 transporters.
- Avoid the use of strong CYP3A4 inhibitors such as P-gp, lopinavir/ritonavir, ritonavir, concomitantly with CYP3A4 inhibitors (eg, conivaptan, ketoconazole), this may increase the risk of bleeding.
- Take care when using P-gp or weak or moderate CYP3A4 inhibitors (eg, erythromycin and azithromycin), together with P-gp.
- Avoid the concurrent use of P-gp or strong CYP3A4 inducers (eg, carbamazepine and phenytoin), as these drugs can decrease the systemic effects of rivaroxaban, and increase the risk of thromboembolic reactions.
- The risk of bleeding increases when you combine other drugs that affect hemostasis. These include aspirin and P2Y12 platelet inhibitions.
- Clopidogrel should not be used in conjunction with other drugs unless there is a clear benefit. The bleeding time increased by approximately twice as much with Clopidogrel alone.
Pregnancy and lactation
Pregnancy Risk Factor: X
The limited data available in pregnant women is insufficient to determine if there is a drug-associated danger of adverse developmental outcomes.
Pregnant women should be cautious as there is a risk of pregnancy-related hemorrhage or emergent birth. Standard laboratory testing cannot reliably monitor anticoagulant effects.
When prescribing medication to pregnant women, consider the risks and benefits for the mother as well as the potential risks to the fetus.
Considerations for clinical purposes
VTE is a risk factor during pregnancy. VTE risk increases for women with inherited and acquired thrombophilias. Pregnant women with thromboembolic diseases have an increased chance of developing maternal complications (eg pre-eclampsia). Maternal thromboembolic diseases increase the risk of intrauterine growth restriction, placental abruption, and early and later pregnancy loss.
Based on the pharmacologic activity and potential to cross the placenta of Factor Xa inhibitors, bleeding can occur at any location in the fetus or neonate.
Anticoagulants are not recommended for pregnant women. Patients who receive anticoagulants during pregnancy, including those in labor, may be at greater risk for bleeding.
Xarelto Use during Breastfeeding:
Human milk contains a drug that has been detected. We don’t have enough data to assess the effects of drugs on infants or milk production. However, we do know that drugs and/or their metabolites are present in rats’ milk.
Take into account the developmental and health benefits that breastfeeding offers, as well as the mother’s need for therapy. Also, consider any possible adverse effects on infants breastfed from therapy or an underlying condition.
Pregnancy Categories
A: Acceptable in general. Studies in pregnant women that have been controlled for fetal risk show no evidence.
B: Acceptable. Either animal studies do not show any risk, but human studies are not available, or animal studies have shown minor risks and human studies did no harm.
C: If the benefits are greater than the risks, use caution. Human studies are not possible or animal studies have not been done.
D: Use when there is no other safer option. Positive evidence of human fetal risk
Pregnancy: Avoid the use of Xarelto in pregnancy. The potential risks outweigh the benefits. Safer alternatives exist.
Pharmacology of Xarelto (Rivaroxaban):
Mechanism of Action (MOA) of Xarelto (Rivaroxaban):
Factor Xa inhibitor inhibits platelet activation. It selectively blocks the active site for factor Xa, without the need for a cofactor (eg antithrombin III).
The blood coagulation cascade depends on the activation of factor Xa through the intrinsic and extrinsic pathways. These pathways play a key role in the blood-coagulation cascade
Evidence of dose-dependent inhibition in factor Xa activities; anti-factor Xa activity can also be influenced by Rivaroxaban; prolongs HepTest and aPTT.
Absorption
Bioavailability: 80-100%
Peak plasma time: 2 – 4 hr
AUC: 29-56% lower when released in the proximal large intestine than with gastric absorption
Distribution
Protein-bound: 92-95% (mainly from albumin).
Vd: 50 L
Metabolism
Metabolized through oxidative decomposition catalyzed CYP3A4/5 or CYP2J2 and also metabolized via hydrolysis
Plasma consists of unchanged rivaroxaban, which is the dominant moiety and has no active or major circulating metabolites (50 percent higher for patients of Japanese descent).
Substrate for P-gp (Bcrp), efflux transporter proteins ABCG2 and P-gp
Elimination
Half-life: 5-9 hr; 11-13 hr (elderly)
Total body clearance: 10 L/hr after IV administration
Excretion: Feces (21% as Metabolites; 28% unchanged), Urine (30% as Metabolites; 36% unchanged).
How to administer Xarelto?
Oral Administration
- 2.5 mg and 10 mg tablets: Can be taken with or without food. Bioavailability is not affected by fasting.
- 15 mg and 20 mg tablets: Take with food. Bioavailability increases when taken with food
Patients who are unable to swallow whole tablets
- Mix the crushed tablets with the applesauce right before you use them
- Take a crushed 2.5-mg tablet or 10-mg tablet with food or both
- Take a crushed 15-mg/20-mg tablet and immediately follow with food
Administration via a Nasogastric feeding tube:
- Take 50mL of water and crush the tablets. Then, administer via a gastric feeding tube or NG.
- Absorption is dependent on the site of drug release in your gastrointestinal tract (gastric or small intestine); avoid administering distal to your stomach as this can reduce the absorption and, thereby, lower drug exposure.
- If you are administering crushed tablets via a tube feeding tube, ensure that the tube is properly positioned.
- Dosage should be administered immediately after swallowing a crushed tablet of 15-mg/20-mg.
In case, you missed the dose …
2.5 mg PO Bid:
- If you miss a dose, take one 2.5-mg dose according to the recommendation at the next scheduled time
15 mg PO Bid:
- Take immediately to ensure that you get 30 mg/day. In this case, two 15-mg tablets can be taken simultaneously. Continue with regular 15 mg Q12hr the next day.
If you are taking 10, 15, or 20, mg PO q Day:
- take the missed dose right away. Do not double-dose within the same day.
Xarelto Storage
Tablets: Keep at room temperature, 25oC (77oF); you can take them with you to 15-30oC (59-86oF).
Crushed tablets in water, or applesauce. Store at room temperature for up to 4 hours.
