Serotonin Modulators are a class of medicines that are different from SSRIs (selective serotonin reuptake inhibitors), SNRIs (selective norepinephrine reuptake inhibitors), MAO inhibitors (monoamine oxidase inhibitors), TCAs (Tricyclic antidepressants), and atypical antidepressants.
The four drugs included in the serotonin modulators are:
- Nefazodone (Serzone)
- Trazodone (Oleptro)
- Vilazodone (Viibryd)
- Vortioxetine (Brintellix)
The serotonin modulators are distinct from other classes of antidepressants that include selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, atypical antidepressants, tricyclics, and monoamine oxidase inhibitors. The serotonin modulators are antagonists and agonists of the postsynaptic receptors for serotonin and can inhibit reuptake to varying degrees. Norepinephrine is not affected.
When to use serotonin modulators?
Before prescribing serotonin modulators, clinicians need to discuss:
- The time required for a clinical response,
- Side effects of these drugs,
- When to discontinue the medication, and
- Important Drug Interactions
When do serotonin modulators start to work?
The effects of serotonin modulators are not seen instantly. Although some response often occurs within the first two weeks of treatment, it may take many weeks (eg, 8 to 14) for a full response depending on the severity of illness and comorbid disease. Comorbid conditions and patients with severe depression respond late to the treatment than usual.
Start with a low dose to prevent side effects, and then increase slowly. Patients with anxiety and depression may be able to tolerate half the recommended dose. The doses are adjusted based on patient response, tolerance, and clinical urgency.
Start with a low dose
Trial and error are required to determine the most effective antidepressant dose. The drug should be started and the dose titrated to the maximum effective dose. Follow-up monitoring of the patient’s response over the next two to four weeks is necessary.
Patients who are unable to tolerate the antidepressant, but still respond well, should continue to increase the dose gradually (to minimize side effects). This is done every two to four weeks. Patients who have recovered from major depression episodes should receive maintenance treatment using the same dose that was used to treat the episode.
Determine the most effective dose via Trial and Error
Side effects of Serotonin Modulators:
Serotonin modulators have some of the same side effects as those of SSRIs and SNRIS. In addition, serotonin modulators are not recommended to be used during pregnancy.
Serotonin Syndrome:
- Serotonin modulators can increase serotonergic neurotransmission and may result in serotonin syndrome. Serotonin syndrome manifests as hyperthermia, sweating, altered mentation, abnormal autonomic symptoms such as fluctuating blood pressure and sweating, and agitation.
Suicide:
- Antidepressants may have a potential impact on suicide ideation and behavior among adults. This is also not uncommon in patients using serotonin modulators.
Important Drug interactions:
- Using serotonin modulators in combination with other drugs can cause a decrease or an increase in drug metabolism. This may require adjusting the dosage or switching to another antidepressant.
- Hepatic cytochrome P450 3A4 or 2D6 (CYP3A4) enzymes are responsible for the metabolism of serotonin modulators. Combining a serotonin modulator with another drug that inhibits them can lead to increased serum levels and toxicities. Likewise, drugs that increase the plasma levels of these enzymes can lead to therapeutic failure and lower serum levels.
- Trazodone And Vilazodone:
- CYP3A4 enzymes extensively metabolize vilazodone and trazodone.
- Vortioxetine:
- Vortioxetine is extensively metabolized by CYP2D6. When vortioxetine has been administered with other medications that inhibit CYP2D6 metabolic pathways or drugs that incite them, drug-drug interactions may occur. CYP2D6 inhibitors are listed in the table.
Strong CYP2D6 Inhibitors - Bupropion
- Fluoxetine
- Dacomitinib
- Paroxetine
- Quinidine
- Tipranavir
Moderate CYP2D6 Inhibitors - Abiraterone
- Cinacalcet
- Darifenacin
- Darunavir
- Duloxetine
- Givosiran
- Lorcaserin
- Mirabegron
- Perhexiline
- Rolapitant
- Terbinafine
- Thioridazine
- Nefazodone:
- Nefazodone appears to undergo extensive metabolism via CYP3A4. Strong CYP3A4 inhibitors and inducers are listed in the table:
Strong CYP3A4 inhibitors | Strong CYP3A$ Inducers |
|
|
- Nefazodone is itself a strong inhibitor of CYP3A4 and can elevate levels of comedications that are dependent upon CYP3A4 for clearance.
Other medications that increase serotonin levels in the central nervous systems can interact with serotonin modulators, potentially leading to serotonin syndrome. These drug-drug interactions, such as with monoamine oxidase inhibitors, can lead to severe side effects.
Serotonin Modulators: Nefazodone (Serzone)
Indications and Uses of Nefazodone:
- Nefazodone is used to treat major depression and premenstrual syndrome. Patients with high serum transaminases or active liver disease or liver injury from previous nefazodone treatments are not recommended to take the drug.
Pharmacology and MOA of Nefazodone:
- It is a phenylpiperazine that has a structure that is similar to that of trazodone. Nefazodone antagonizes and down-regulates postsynaptic serotonin 5-HT2A receptors, and weakly inhibits presynaptic serotonin and norepinephrine reuptake. The drug has little effect on dopamine D2, alpha-adrenergic and cholinergic receptors.
Nefazodone Dose, and Administration:
- 100 mg twice daily is the usual starting dose for major depression. Patients who fail to respond within two to four weeks are given 150 to 200 mg twice daily. To achieve the desired clinical response, the dose can be increased by increasing the dose by 100 to 200mg per day for two to four weeks. The maximum daily dose is 300mg twice daily. Dose increases may be made once per week in clinically critical situations. The effectiveness and tolerability of once-daily and twice-daily dosing may be comparable.
- If nefazodone has been prescribed with a strong inhibitor CYP3A4, it may be necessary to decrease the dose. If nefazodone has been given concurrently with a strong inducer, however, the dose may need to increase.
How to discontinue Nefazodone:
- Although abrupt discontinuation of nefazodone does not cause a withdrawal syndrome, it is best to taper the drug over one week before stopping it, which is consistent with the preferred method of discontinuing any psychotropic medication.
Side effects of Nefazodone:
- Nefazodone may cause liver damage; an estimate of the incidence of hepatotoxicity, based on a Spanish national registry, is 29 per 100,000 patients annually. Adverse hepatic reactions can occur with doses as low as 100 mg per day and generally occur within six months of starting the drug [Ref].
- These reactions include acute liver failure; the estimated incidence is 1 case per 200,000 to 300,000 patient-years, which is three to four times greater than expected. A 2010 study of the World Health Organization Programme for International Drug Monitoring database found 94 cases of acute liver failure attributed to nefazodone, including patients who received a liver transplant or died [Ref].
- The generic version of nefazodone that is available in the United States is no longer produced. The drug was also pulled from several countries. Patients who are taking the drug should be closely monitored for symptoms such as nausea, abdominal pain, jaundice, and impaired synthetic function. Although periodic liver function tests (eg, every two to six months) may possibly be useful, there is no evidence that testing prevents hepatic injury.
- Nefazodone can cause several other side effects. A pooled analysis of randomized trials (n = 2185 patients, most with unipolar major depression) found that the incidence of discontinuing nefazodone because of side effects was 12 percent and for placebo was 7 percent [Ref].
- Other common adverse drug reactions include Nausea, sleepiness, and dry mouth. Most of these side effects are dose-related.
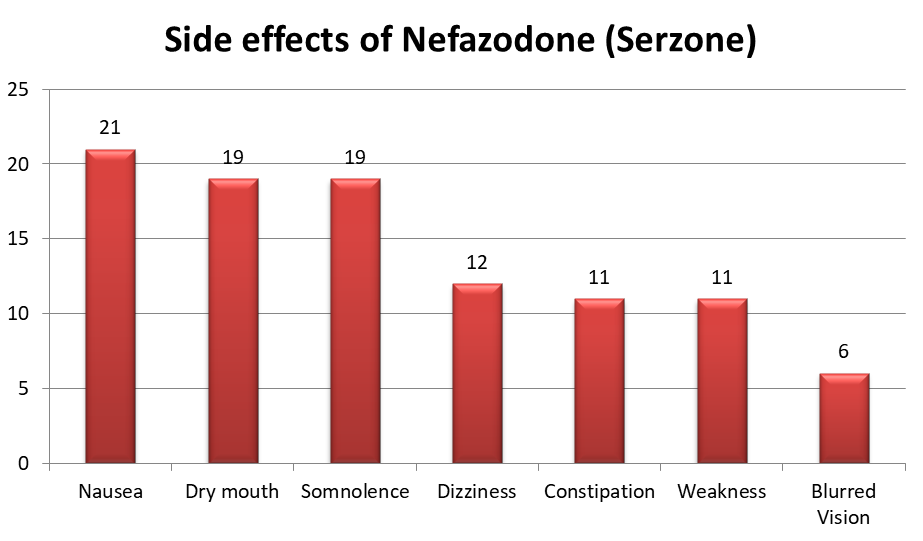
- Uncommon side effects of Nefazodone include a drop in systolic blood pressure, sinus bradycardia, dryness, and confusion. Compared to other SSRIs, it does not cause significant weight gain and is not associated with sexual dysfunction.
Serotonin Modulator: Trazodone (Oleptro)
Trazodone (Oleptro) Uses and indications:
- Trazodone is used to treat major depression as well as functional dyspepsia. It is also used to treat insomnia caused by antidepressants such as bupropion and fluoxetine. The effectiveness of trazodone to treat insomnia without depression seems to be limited.
Trazodone (Oleptro ) MOA:
- Trazodone has a triazolopyridine structure that resembles nefazodone. Trazodone is a triazolopyridine that acts on postsynaptic 5-HT2A/5HT2C receptors.
- It also weakly inhibits presynaptic reuptake of serotonin. The effects appear to be dose dependent such that at low doses the drug acts as a serotonin antagonist and at high doses as a serotonin agonist.
- The drug has minimal effects on dopamine and norepinephrine reuptake. In addition, the drug blocks postsynaptic alpha-adrenergic receptors (which may account for the side effects of orthostatic hypotension and priapism) and histamine H1 receptors (which may explain its sedative effect). The drug does not have an effect on cholinergic receptors.
Trazodone Dose and Administration:
- For major depression, Trazodone immediate delivery is usually administered as follows:
- Start with 50 mg twice daily.
- Then, the drug is increased in increments of 50 mg per day for three to seven days until it reaches a daily dose of 75-150 mg twice daily.
- After achieving the desired clinical response, the dose is increased by 50-100 mg per day for two to four weeks to reach a maximum of 600 mg daily.
- Patients who consume more than 400 mg per day should be monitored and cautioned. Patients may tolerate the drug’s sedative effects better if they are given a lower daytime dose and a higher bedtime dose (e.g. 100 mg in morning, 200 mg at night). Some patients get the entire dose at bedtime. Extended release preparations are available in some countries but not in the United States.
- Patients suffering from insomnia due to antidepressants (eg selective serotonin receptor inhibitors) might benefit from immediate release of trazodone 50-100 mg at bedtime. When adjunctive trazodone is prescribed as a hypnotic for insomnia associated with depression, doses typically range from 50 to 300 mg at bedtime.
- For major depression, Trazodone immediate delivery is usually administered as follows:
For the treatment of Insomnia, the dose rarely exceeds 200 mg.
Trazodone Drug Interactions:
- Trazodone undergoes extensive hepatic metabolism by hepatic cytochrome P450 3A4 (CYP3A4) enzymes. A stronger inhibitor of CYP3A4 may be prescribed with trazodone. The dose of trazodone that is administered concurrently with strong inducers may need to increase. Strong CYP3A4 inhibitors and inducers are listed in the table above.
How to Discontinue Trazodone (Oleptro)?
- Tapering the drug for two to four weeks before discontinuation is recommended. Withdrawal symptoms may include anxiety, gastrointestinal distress, and sleep disturbances if trazodone is abruptly stopped.
Side effects of Trazodone (Oleptro):
- Trazodone may cause side effects. A randomized trial that compared trazodone immediate release with placebo in 153 patients with major depression found that discontinuation of treatment due to side effects was greater in patients who received trazodone than placebo (23 versus 4 percent); trazodone caused a higher incidence of [Ref]:

- Trazodone can also cause headaches and orthostatic hypotension. Rare but serious side effects of trazodone include:
- Priapism (penile erection):
- Penile priapism secondary trazodone should be treated immediately by a physician (rare cases have been reported of clitoral or priapism). Trazodone-induced priapism can occur in 1 to 1000 to 1 to 10,000 patients. It has been reported at doses of 50 to 400 mg daily.
- Priapism can occur up to 18 years after the onset of treatment. Most cases are seen within the first month. Priapism may develop if there is a prolonged period of erection.
- Cardiac arrhythmias:
- A review identified cases reports of atrial or ventricular arrhythmias in patients who received trazodone. Patients with heart disease should not take the drug.
- Trazodone effect on weight:
- Trazodone appears to be weight neutral during treatment lasting 4 to 12 weeks, based upon a meta-analysis of three heterogeneous randomized trials (n = 155 patients treated with trazodone for with major depression).
Read: Which Antidepressant Does Not Cause Weight Gain
Trazodone Overdose:
- Trazadone alone is not usually fatal. A study of 35 patients with doses of up to 6400mg and another of 22 patients with doses of up to 3500mg found no deaths. One fatal suicide involving trazodone has been reported. The patient suffered from torsades, complete atrioventricular dysfunction, multiple organ failure, and a serum concentration of 25 mcg/mL.
- Although most patients recover uneventfully, overdose can cause arrhythmias, respiratory arrest, coma, and priapism.
- In addition, overdoses with trazodone plus alcohol and/or other drugs are often lethal. A study of 49 patients found that 9 (18 percent) died [Ref].
Serotonin Modulator: Vilazodone (Viibryd)
Vilazodone (Viibryd) Uses and Indications:
- Vilazodone is used to treat major depression.
Vilazodone (Viibryd) Uses and Indications:
- Vilazodone, an indolalkylamine, inhibits presynaptic reuptake serotonin. It also acts as a partial agonist for postsynaptic 5-HT1A receptors. There is little inhibition of dopamine and norepinephrine reuptake.
Vilazodone (Viibryd) Dose and Administration:
- For major depression, the usual starting dose is 10 mg daily at bedtime for one week. The dose is then increased to 20 mg per day for week 2. This schedule of titration is designed to reduce gastrointestinal toxicities. The daily target dose should be between 20 and 40 mg.
- To increase bioavailability, the drug should be taken with food. Patients with severe renal impairment do not require dosage adjustments. The drug has not been tested in patients with severe liver impairment.
- Concurrent use of vilazodone with other medications that inhibit or induce hepatic cytochrome P450 3A4 (CYP3A4) metabolism can alter vilazodone serum concentrations, which may necessitate adjusting the dose of vilazodone.
- Recommendations for specific vilazodone dose adjustments are as follows:
- *Vilazodone given with strong CYP3A4 inhibitors – Vilazodone dose should not exceed 20 mg once daily. You can adjust the dose of vilazodone to your original dose if you have stopped using the CYP3A4-inhibitor.
- Vilazodone given with strong CYP3A4 inducers:
- Based upon clinical response, clinicians may need to increase the vilazodone dose two-fold when co-administered with CYP3A4 inducer for more than 14 days. The maximum daily intake of vilazodone can be 80 mg. Reduce the vilazodone dosage to the original dose if the CYP3A4 inhibitor is stopped. This can be done for 7 to 14 days.
How to discontinue Vilazodone (Viibryd)?
- While withdrawal symptoms from abrupt discontinuation have not been reported, we taper the dose of 20-40 mg per day for up to two weeks before discontinuing. Tapering is the preferred method to stop any psychotropic medication.
Side effects of Vilazodone (Viibryd):
- Vilazodone can have side effects. A pooled analysis examined adverse effects in two randomized trials that compared vilazodone 40 mg per day with placebo for eight weeks in 891 patients with unipolar major depression; the following adverse effects occurred more often in patients who received vilazodone than placebo, and the incidence with vilazodone was as follows [Ref]:
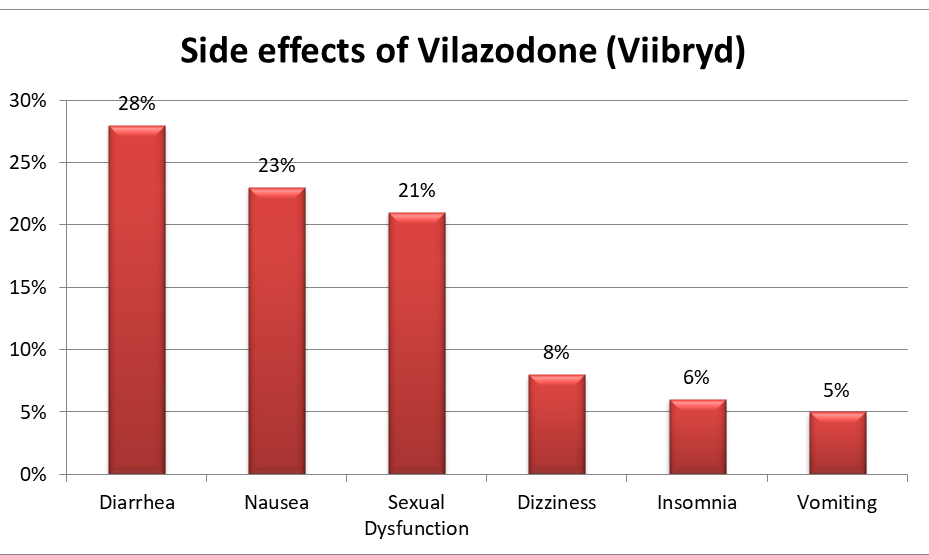
- Patients treated with vilazodone have reported the potentially life threatening serotonin syndrome. There is also a concern about the possibility that maternal use of vilazodone during pregnancy as it may cause persistent pulmonary hypertension of the newborn.
Serotonin Modulator: Vortioxetine (Brintellix)
Vortioxetine (Brintellix) Uses and Indications:
- Vortioxetine is used to treat major depression. Vortioxetine may also improve cognitive function in patients suffering from unipolar major depressive disorder (eg duloxetine). This benefit may not be related to the resolution of the depressive syndrome.
Vortioxetine MOA:
- Vortioxetine, a bis-arylsulphanylamine that inhibits serotonin presynaptic uptake is the main mechanism behind the drug’s antidepressant effects. In addition, vortioxetine interacts with several serotonin receptor subtypes; the medication is a potent antagonist at serotonin 5-HT3 receptors, a weaker antagonist at 5-HT7 and 5-HT1D receptors, a partial agonist at 5-HT1B receptors, and a full agonist at 5-HT1A receptors.
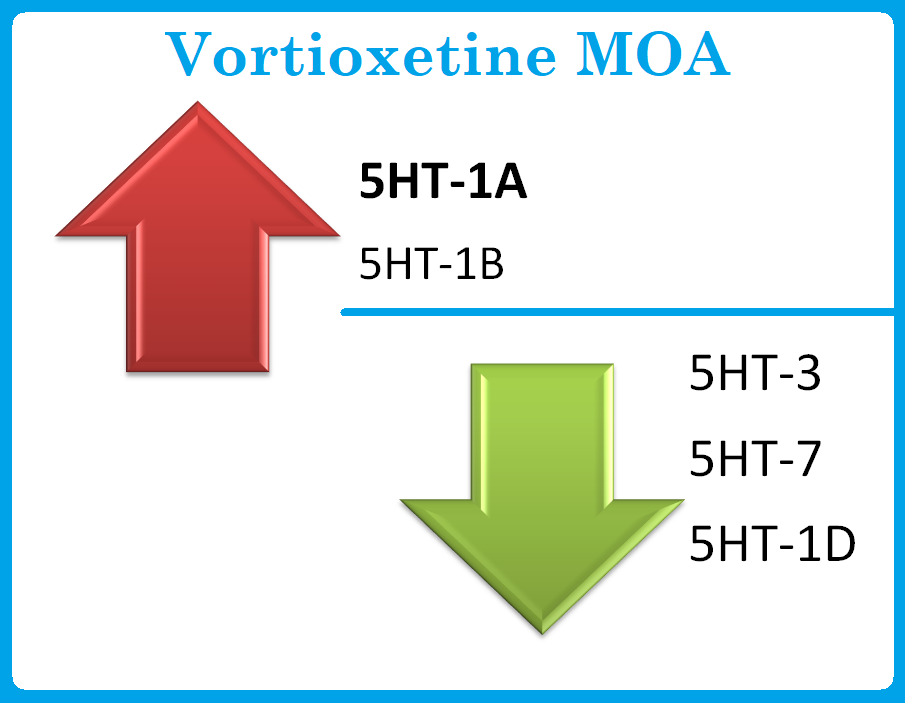
- The downstream pharmacodynamic effects include increased levels of serotonin, acetylcholine, dopamine, and norepinephrine in specific areas of the brain. Although it is not known if the drug has any clinical effects on subtypes of serotonin receptors, these effects could possibly mediate the drug’s therapeutic benefits as well as its side effects.
Vortioxetine dose and Administration:
- It is recommended that vortioxetine be started at 10 mg daily. Patients who are able to tolerate the drug during week 1 can be increased to 20 mg daily.
- An alternative to starting treatment is to start with 5 mg/day in week 1. Then, you can titrate to 10 mg/day week 2. Week 3: 20 mg/day or 15 mg/day week 3. Week 4: 20 mg/day.
- It is also sensible to test the efficacy of each dose for at least two to four weeks before increasing the dosage.
- Dose adjustments are not required for older patients (eg, age >=65 years) and for patients with renal impairment or mild to moderate hepatic impairment.
- Vortioxetine undergoes extensive metabolism by hepatic cytochrome P450 2D6 (CYP2D6) enzymes and is also metabolized by other CYP enzymes. Patients who slow metabolize CYP2D6 substances should take 10 mg/day.
- Patients who have a slow metabolism of CYP2D6 substrates may need to adjust their doses.
How to discontinue Vortioxetine (Britellix, Trintellix)?
- While abrupt discontinuation does not cause withdrawal symptoms, it is recommended to taper the dose of vortioxetine from 15 to 20 mg/day to 10 mg/day for one week. Tapering is the preferred method of stopping any psychotropic medication.
Side effects of Vortioxetine (Brintellix, Trintellix):
- Vortioxetine can cause nausea, but the drug is generally well tolerated. However, compared to placebo, the rates of treatment discontinuation were greater in the vortioxetine group vs placebo due to the side effects.
- Nausea is reportedly the most common side effect [Ref].

- Other common side effects of vortioxetine vs placebo include vomiting, and constipation. The drug has little effects on laboratory parameters, vital signs, and electrocardiogram.
- Studies have shown that side effects of vortioxetine are less common than those of other antidepressants like duloxetine and agomelatine.
Vortioxetine Overdose:
- Vortioxetine overdose is only known from clinical trials where subjects consumed between 40 and 75 mg of the drug. This was associated with increased nausea, vomiting, diarrhea, cramping, pruritus and flushing.
