Bempedoic acid is a novel class of medicine that has got FDA approval in February 2020. It is used to treat patients with hyperlipidemia who do not achieve their target lipid levels despite maximally tolerated doses of statins.
Bempedoic acid Uses:
It is approved for the treatment of patients who have an established cardiovascular atherosclerotic disease and who require lowering of LDL levels and as an adjunct to diet, exercise, and maximally tolerated statins.
It is also used in patients with familial heterozygous hypercholesterolemia as an adjunct to diet and exercise and maximally tolerated statin therapy who require additional lowering of LDL levels.
Bempedoic Acid efficacy in Landmark Clinical Trials:
The two major trials that showed the efficacy of Bempedoic acid were the “Clear Harmony” and “Clear Wisdom” Trials. In both these trials, Bampedoic acid significantly reduced LDL levels and was well tolerated.
The “Clear Harmony Trial” [Ref]
The “Clear Harmony Trial” was a double-blinded randomized clinical trial that involved a total of 2230 patients. All patients had either atherosclerotic cardiovascular disease or familial heterozygous hyperlipidemia and a baseline LDL of greater than 70 mg/dl despite maximally tolerated statin therapy. At week 12, Bempedoic acid significantly reduced the LDL by 19.2 mg/dl (16.5% change from baseline). The risk of adverse drug reactions was greater than placebo especially gout but was not statistically significant.
The “Clear Wisdom Trial” [Ref]
The “Clear Wisdom Trial” was a placebo-controlled double-blinded randomized controlled phase 3 clinical trial that included a total of 779 patients with established cardiovascular disease, heterozygous familial hypercholesterolemia, or both and despite maximally tolerated statins, had LDL levels exceeding 70 mg/dl.
At week 12, Bempedoic Acid significantly reduced LDL, 17.4% reduction versus placebo [95% CI, –21.0% to –13.9%]; P < .001). It also significantly reduced total cholesterol, apolipoprotein B, and high sensitive CRP levels.
Bempedoic acid Drug Class: Adenosine Triphosphate-Citrate Lyase (ACL) Inhibitor
It is a novel class of medicine that works upstream of the HMG CoA reductase (statins) in the cholesterol biosynthesis pathway.
MOA (Mechanism of action of Bempedoic acid):
Bempedoic acid acts in the liver upstream of statins in the cholesterol biosynthesis pathway. It is an inhibitor of adenosine triphosphate-citrate lyase (ACL). It reduces the synthesis of cholesterol in the liver.
The drug is converted into its active metabolite ESP15228 within the liver. Both the prodrug and its active metabolite further require activation by very long-chain acyl-CoA synthetase 1 (ACSVL1) to ETC-1002-CoA and ESP15228-CoA, respectively. After activation, it reduces the synthesis of cholesterol in the liver and decreases the LDL-C receptors in the blood.
Side effects of Bepedoic Acid:
The most common or significant side of the drug, that was observed in clinical trials was hyperuricemia and Gout. The effect on hyperuricemia is dose-related and is thought to occur as a result of the inhibition of OAT2 (Organic anion transporter 2). Hyperuricemia may occur early in the course of the disease (within four weeks) and may persist throughout the treatment period. Patients with a history of gout are especially at a high risk of developing a flare of gout and hyperuricemia.
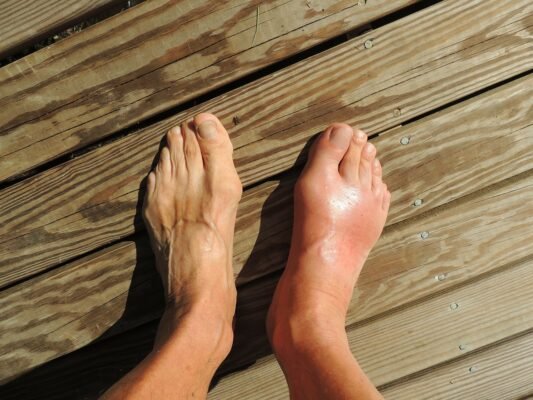
Another significant side of the drug is tendon rupture that may involve the rotator cuff muscles, biceps tendon, or the Achilles tendon. Patients may develop tendon injuries at any time during the study period. Elderly patients (those aged 60 or more), patients on concomitant corticosteroids or fluoroquinolones, patients with kidney disease, and those with a prior history of tendon rupture are at a greater risk of developing tendon rupture when initiated on Bempedoic acid treatment.
Other side effects of Bempedoi acid include nasopharyngitis, blood disorders (leukopenia, anemia, thrombocytopenia, abdominal pain and distension, atrial fibrillation, and raised creatine kinase.
It may also cause an elevation of hepatic enzymes, urea, and creatinine. It has also been associated with muscle and back pain
Brand names of Bempedoic Acid: Nexletol
Bempedoic Acid Dose:
The usual dose in both the approved conditions is 180 mg once daily.
The Maximum Daily Dose is 180 mg once daily.
Monitoring Parameters:
Lipid parameters should be monitored after 8 to 12 weeks of treatment initiation. All patients should be assessed for the risks of developing tendon rupture. Patients should be closely monitored for hyperuricemia and flare of gout during the treatment.
